Automated workflow for high throughput quality control using LC/GC-MS
Compound quality control can be challenging and time consuming when manual input for processing or result interpretation is needed. If you are an organic chemist, medicinal chemist, formulation scientist, or a quality specialist and want to analyze your compound libraries to assert compound identity and assess purity, Chrom QC is your best ally. This automation tool will run end-to-end QC workflows for you and deliver decision-enabling reports with all the results you’ll need.
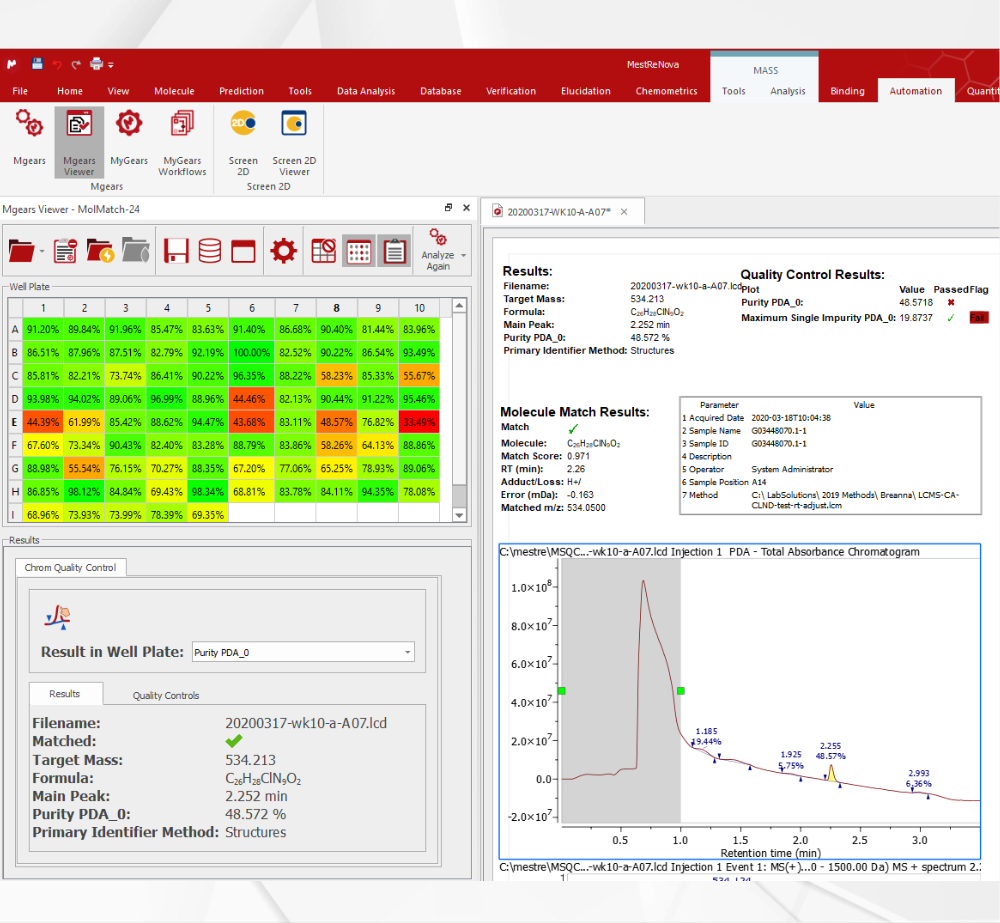
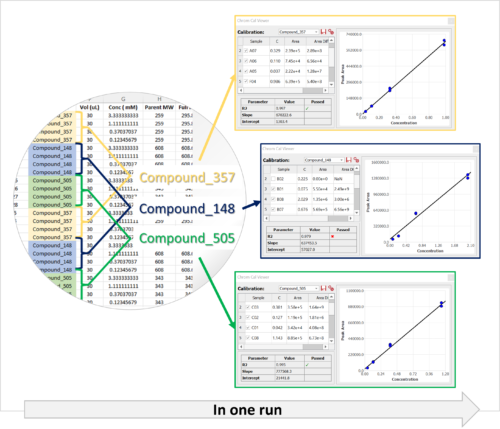
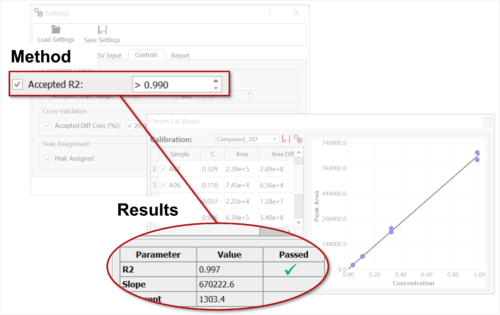
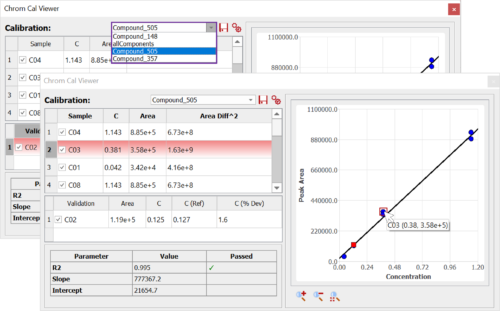
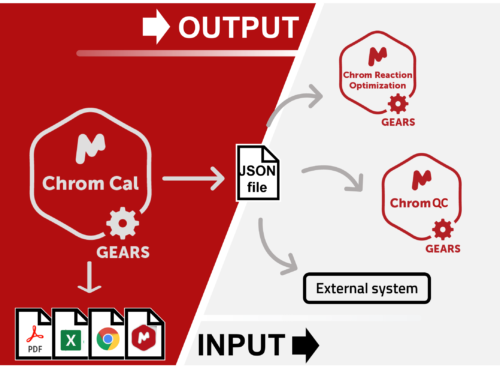
Chrom QC runs on the Gears automation engine, which means that any LC/GC-MS format can be handled independently from the instrument software packages. Data is automatically retrieved from your directories (in batches or real time), matched to the corresponding molecular structures, formulae, masses, etc., and analyzed to confirm compound identity and determine purities. Custom csv, html, mnova, and PDF reports can also be automatically generated, and results can be sent directly to your database! Even better, all of this can be achieved with only minimal setup effort!
Chrom QC
Benefits
More Automation
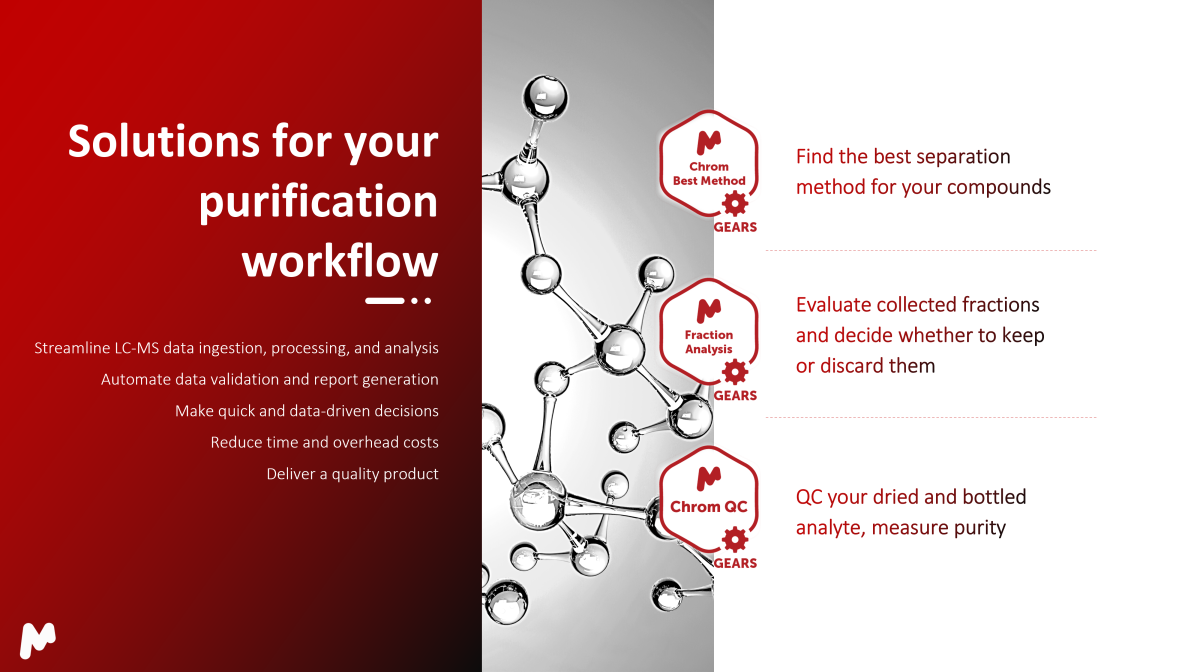
Purification Workflow
Looking for an enterprise-level solution for improving your purification and data management procedures? Check the Best Method and Fraction Analysis bricks that also run in full automation, and learn more about our automated databasing solution with Mgears. You have the complete flexibility to combine and adapt these solutions to your standard procedures to reap even more benefits for your organization.
Contact us for more information.
Academic, Government & Industrial
Markets
Who should be using Chrom QC?
Organic chemists, analysts, QC experts, and researchers using quantitative chromatographic methods for quality control, concentration/purity determination, or monitoring/optimization of reactions in one of the following markets and applications:
- Pharmaceutical/Drug development industry
- Food & Beverage industries
- Personal care and Cosmetics
- Fine chemical synthesis industries
- Polymer industries
- Academic research laboratories
- Contract Research Organizations (CROs)
- Quality control of synthesized or acquired compounds prior to registration
- Quality control of compound collections prior to use in biological assays
- Quality control of finished compounds prior release to the market (drugs, food additives, preservatives, etc.)
- Quality control for formulation optimization and definition of storage recommendations
Proyecto SmartGlobalLab: Un mercado global de datos farmacéuticos (Nº 2021/C005/00150505) de la Convocatoria de ayudas 2021 destinadas a proyectos de investigación y desarrollo en inteligencia artificial y otras tecnologías digitales y su integración en las cadenas de valor (C005/21-ED), impulsado por Red.es y realizado bajo el Plan de Recuperación, Transformación y Resiliencia – Financiado por la Unión Europea – NextGenerationEU.
SmartGlobalLab Project: A global pharmaceutical data marketplace (No. 2021/C005/00150505) under the 2021 Call for grants for research and development projects in artificial intelligence and other digital technologies and their integration into value chains (C005/21-ED), promoted by Red.es and carried out under the Recovery, Transformation and Resilience Plan – Funded by the European Union – NextGenerationEU.